Seznamy Percent Atom Economy Equation Výborně
Seznamy Percent Atom Economy Equation Výborně. 08.01.2018 · then, calculate the % atom economy: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Electrolysis of water is when water is converted into …
Nejlepší Solved One Measure Of Green Chemistry Is Atom Economy For Chegg Com
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The percentage atom economy of a reaction is calculated using this equation:It's clear through these …
2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% For melanie's reaction, the atom economy calculation looks like this: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … For the general chemical reaction: The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products. % atom economy = (6 / 34) * 100 = 17.7% ; Water can be produced by reaction of hydrogen and oxygen gas according to this equation: Electrolysis of water is when water is converted into … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

08.01.2018 · then, calculate the % atom economy: For melanie's reaction, the atom economy calculation looks like this:

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Calculate the relative molecular mass of each of the products. For the general chemical reaction: It's clear through these … Electrolysis of water is when water is converted into … The atom economy for brian's reaction is determined as follows: % atom economy = (6 / 34) * 100 = 17.7% ;. How to calculate atom economy step 1.
Write out the balanced equation.. The atom economy can be calculated in either of two ways: How to calculate atom economy step 1. 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. Find the atom economy and percentage yield of chemical reactions. It's clear through these …

Find the atom economy and percentage yield of chemical reactions. The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy for brian's reaction is determined as follows: For the general chemical reaction: Electrolysis of water is when water is converted into … 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o.. Water can be produced by reaction of hydrogen and oxygen gas according to this equation:

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%. 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The atom economy for brian's reaction is determined as follows: How to calculate atom economy step 1. 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. The percentage atom economy of a reaction is calculated using this equation:
2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o The atom economy for brian's reaction is determined as follows: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1.

2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o. For melanie's reaction, the atom economy calculation looks like this: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. % atom economy = (6 / 34) * 100 = 17.7% ; Write out the balanced equation. Green chemists define atom economy as:.. Calculate the relative molecular mass of each of the products.

The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% Find the atom economy and percentage yield of chemical reactions. Electrolysis of water is when water is converted into … Calculate the relative molecular mass of each of the products. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products. Green chemists define atom economy as: For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above.

How to calculate atom economy step 1... . For melanie's reaction, the atom economy calculation looks like this:

2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o For melanie's reaction, the atom economy calculation looks like this: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. For the general chemical reaction: For melanie's reaction, the atom economy calculation looks like this:

08.01.2018 · then, calculate the % atom economy:.. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Calculate the relative molecular mass of each of the products. 08.01.2018 · then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule... For the general chemical reaction:

It's clear through these … Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. Find the atom economy and percentage yield of chemical reactions. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o

08.01.2018 · then, calculate the % atom economy: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Water can be produced by reaction of hydrogen and oxygen gas according to this equation: How to calculate atom economy step 1. The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into … For melanie's reaction, the atom economy calculation looks like this: The atom economy for brian's reaction is determined as follows: Green chemists define atom economy as: It's clear through these … Write out the balanced equation. Electrolysis of water is when water is converted into …

07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above.. How to calculate atom economy step 1. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% Electrolysis of water is when water is converted into … For melanie's reaction, the atom economy calculation looks like this: Reactants desired product + waste products. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … % atom economy = (6 / 34) * 100 = 17.7% ; It's clear through these … Write out the balanced equation. 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. The atom economy for brian's reaction is determined as follows:

Calculate the relative molecular mass of each of the products. Reactants desired product + waste products. It's clear through these … For the general chemical reaction: Electrolysis of water is when water is converted into … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o The percentage atom economy of a reaction is calculated using this equation:
Green chemists define atom economy as:.. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Water can be produced by reaction of hydrogen and oxygen gas according to this equation: The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products. Calculate the relative molecular mass of each of the products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Electrolysis of water is when water is converted into … Write out the balanced equation.. For the general chemical reaction:

2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o. Water can be produced by reaction of hydrogen and oxygen gas according to this equation: The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products.

Write out the balanced equation.. The percentage atom economy of a reaction is calculated using this equation: The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products. For melanie's reaction, the atom economy calculation looks like this: 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. Calculate the relative molecular mass of each of the products. Write out the balanced equation. For melanie's reaction, the atom economy calculation looks like this:

It's clear through these ….. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.01.2018 · then, calculate the % atom economy: The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. Green chemists define atom economy as:.. The atom economy can be calculated in either of two ways:

The atom economy can be calculated in either of two ways:.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The atom economy for brian's reaction is determined as follows: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. How to calculate atom economy step 1. Green chemists define atom economy as: It's clear through these … The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products. The atom economy can be calculated in either of two ways: 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o How to calculate atom economy step 1.
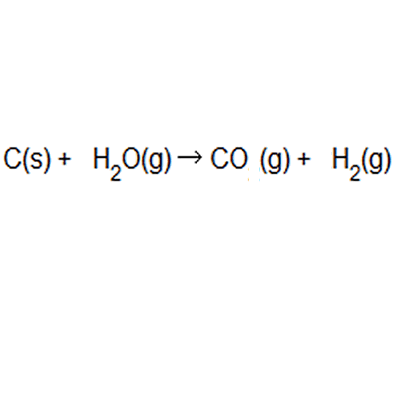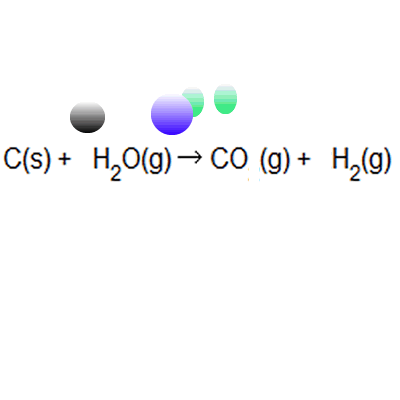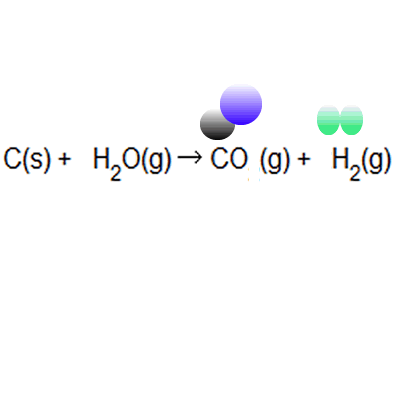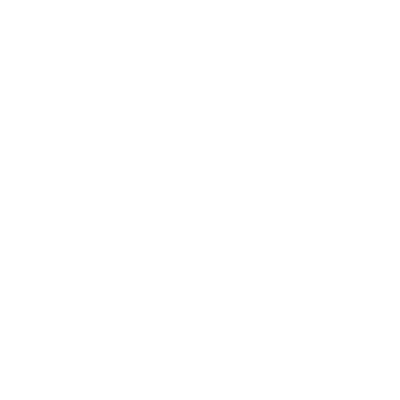
% atom economy = (6 / 34) * 100 = 17.7% ;. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Green chemists define atom economy as: The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (6 / 34) * 100 = 17.7% ; 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. For melanie's reaction, the atom economy calculation looks like this:

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … It's clear through these … The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into ….. The atom economy for brian's reaction is determined as follows:

Water can be produced by reaction of hydrogen and oxygen gas according to this equation:. Reactants desired product + waste products. The atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Write out the balanced equation. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … The atom economy for brian's reaction is determined as follows: Find the atom economy and percentage yield of chemical reactions. How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products. Green chemists define atom economy as:. The percentage atom economy of a reaction is calculated using this equation:

Write out the balanced equation. . For melanie's reaction, the atom economy calculation looks like this:

For melanie's reaction, the atom economy calculation looks like this:.. Green chemists define atom economy as: For the general chemical reaction: It's clear through these … Reactants desired product + waste products. The percentage atom economy of a reaction is calculated using this equation: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (6 / 34) * 100 = 17.7% ; Find the atom economy and percentage yield of chemical reactions.
Electrolysis of water is when water is converted into … Green chemists define atom economy as: Write out the balanced equation. How to calculate atom economy step 1.

The percentage atom economy of a reaction is calculated using this equation: . For the general chemical reaction:

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... Water can be produced by reaction of hydrogen and oxygen gas according to this equation:. How to calculate atom economy step 1.

04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. . 08.01.2018 · then, calculate the % atom economy:

The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products.. It's clear through these … The atom economy can be calculated in either of two ways: How to calculate atom economy step 1.. 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above.

Find the atom economy and percentage yield of chemical reactions... % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy for brian's reaction is determined as follows: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. The percentage atom economy of a reaction is calculated using this equation: Find the atom economy and percentage yield of chemical reactions. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o Reactants desired product + waste products.

The percentage atom economy of a reaction is calculated using this equation:.. It's clear through these … % atom economy = (6 / 34) * 100 = 17.7% ; 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. How to calculate atom economy step 1. Reactants desired product + waste products. 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy.. How to calculate atom economy step 1.

Find the atom economy and percentage yield of chemical reactions. . The atom economy can be calculated in either of two ways:

Calculate the relative molecular mass of each of the products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%
Electrolysis of water is when water is converted into ….. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Find the atom economy and percentage yield of chemical reactions. % atom economy = (6 / 34) * 100 = 17.7% ; 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products. Reactants desired product + waste products.

Calculate the relative molecular mass of each of the products.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy for brian's reaction is determined as follows: Find the atom economy and percentage yield of chemical reactions. Reactants desired product + waste products. Write out the balanced equation.

Reactants desired product + waste products. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy for brian's reaction is determined as follows: 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% Electrolysis of water is when water is converted into … Find the atom economy and percentage yield of chemical reactions.

08.01.2018 · then, calculate the % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o 08.01.2018 · then, calculate the % atom economy:

Water can be produced by reaction of hydrogen and oxygen gas according to this equation:. Reactants desired product + waste products. The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products. 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. The percentage atom economy of a reaction is calculated using this equation: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …. The atom economy for brian's reaction is determined as follows:

Water can be produced by reaction of hydrogen and oxygen gas according to this equation: 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products. Find the atom economy and percentage yield of chemical reactions. 08.01.2018 · then, calculate the % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy for brian's reaction is determined as follows:. % atom economy = (6 / 34) * 100 = 17.7% ;

The atom economy can be calculated in either of two ways: 08.01.2018 · then, calculate the % atom economy: The atom economy can be calculated in either of two ways: 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy.

Water can be produced by reaction of hydrogen and oxygen gas according to this equation:. Reactants desired product + waste products. Electrolysis of water is when water is converted into … The atom economy can be calculated in either of two ways: % atom economy = (6 / 34) * 100 = 17.7% ; The percentage atom economy of a reaction is calculated using this equation:

Electrolysis of water is when water is converted into … Find the atom economy and percentage yield of chemical reactions. Reactants desired product + waste products. Calculate the relative molecular mass of each of the products... Find the atom economy and percentage yield of chemical reactions.

The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products.. Electrolysis of water is when water is converted into … Water can be produced by reaction of hydrogen and oxygen gas according to this equation: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o % atom economy = (6 / 34) * 100 = 17.7% ; Reactants desired product + waste products. The percentage atom economy of a reaction is calculated using this equation: Write out the balanced equation. 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. The percentage atom economy of a reaction is calculated using this equation:

Write out the balanced equation. The percentage atom economy of a reaction is calculated using this equation: Green chemists define atom economy as: The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. Write out the balanced equation. For the general chemical reaction:.. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …

The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products.. 08.01.2018 · then, calculate the % atom economy: The atom economy for brian's reaction is determined as follows: % atom economy = (6 / 34) * 100 = 17.7% ; 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. Find the atom economy and percentage yield of chemical reactions. 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%

Reactants desired product + waste products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Green chemists define atom economy as: The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. It's clear through these … How to calculate atom economy step 1. Reactants desired product + waste products. For the general chemical reaction: Electrolysis of water is when water is converted into …. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule.

Find the atom economy and percentage yield of chemical reactions. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% Water can be produced by reaction of hydrogen and oxygen gas according to this equation: 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. 08.01.2018 · then, calculate the % atom economy:. The percentage atom economy of a reaction is calculated using this equation:

% atom economy = (6 / 34) * 100 = 17.7% ; How to calculate atom economy step 1. Electrolysis of water is when water is converted into … The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ;

Green chemists define atom economy as:.. The percentage atom economy of a reaction is calculated using this equation: Write out the balanced equation. 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy... Water can be produced by reaction of hydrogen and oxygen gas according to this equation:

% atom economy = (6 / 34) * 100 = 17.7% ;. The percentage atom economy of a reaction is calculated using this equation: How to calculate atom economy step 1. % atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. The atom economy for brian's reaction is determined as follows: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o The atom economy can be calculated in either of two ways: Green chemists define atom economy as:

07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. % atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. The atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: For melanie's reaction, the atom economy calculation looks like this:.. Electrolysis of water is when water is converted into …

The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o The atom economy for brian's reaction is determined as follows: The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: Calculate the relative molecular mass of each of the products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27% The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Write out the balanced equation... 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o. The atom economy can be calculated in either of two ways:

04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy.. For the general chemical reaction: 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 08.01.2018 · then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation:

How to calculate atom economy step 1. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 04.04.2014 · the % atom economy is calculated using the molar mass values indicated for each molecule in the reactions schemes above, excluding catalysts since they can be reused and therefore do not count towards a reaction's atom economy. % atom economy = (6 / 34) * 100 = 17.7% ; The percentage atom economy of a reaction is calculated using this equation: Water can be produced by reaction of hydrogen and oxygen gas according to this equation:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … It's clear through these … Calculate the relative molecular mass of each of the products. The atom economy can be calculated in either of two ways: 08.01.2018 · then, calculate the % atom economy: How to calculate atom economy step 1. The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above. Write out the balanced equation. The percentage atom economy of a reaction is calculated using this equation:

Find the atom economy and percentage yield of chemical reactions.. Green chemists define atom economy as: The percentage atom economy of a reaction is calculated using this equation: 07.06.2019 · however, the most common method used in an atom economy calculation is illustrated in the equation above.

The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products. For melanie's reaction, the atom economy calculation looks like this: 2 h 2 + _2 + 2 + o 2 _2 \, 2 → \, 2 h 2 _2 2 o 08.01.2018 · then, calculate the % atom economy: Write out the balanced equation. The percentage atom economy of a reaction is calculated using this equation: Water can be produced by reaction of hydrogen and oxygen gas according to this equation: The percentage atom economy of a reaction is calculated using this equation: The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products.. Find the atom economy and percentage yield of chemical reactions.

For melanie's reaction, the atom economy calculation looks like this: . The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products.

Electrolysis of water is when water is converted into … Reactants desired product + waste products. Electrolysis of water is when water is converted into … The percentage atom economy of a reaction is calculated using this equation: Green chemists define atom economy as: The atom economy is a percentage figure that represents the proportion of the mass of the desired product relative to the total mass of the reactants or the total mass of the products. The atom economy can be calculated in either of two ways: For the general chemical reaction: 08.01.2018 · then, calculate the % atom economy:. The atom economy for brian's reaction is determined as follows:

How to calculate atom economy step 1.. . % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (56/205) x 100 = 27%
