Seznamy Atom Quantum Numbers Zdarma
Seznamy Atom Quantum Numbers Zdarma. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. In other words, it refers to … The value of n ranges from 1 to the shell containing the outermost electron of that atom.
Nejlepší Quantum Numbers And Rules Physics
An electron in an atom or ion has four quantum numbers to describe its state. To completely describe an electron in an atom, four quantum numbers are needed: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. N = 1, 2, 3, …, 8. Each electron in an atom is described by four different quantum numbers.14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e.
17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: An electron in an atom or ion has four quantum numbers to describe its state. Each electron in an atom is described by four different quantum numbers. 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. N = 1, 2, 3, …, 8.

A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers... The only information that was important was the size of the orbit, which was described by the n quantum number. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. An electron in an atom or ion has four quantum numbers to describe its state. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Each electron in an atom is described by four different quantum numbers. To completely describe an electron in an atom, four quantum numbers are needed: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus... 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:
14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. To completely describe an electron in an atom, four quantum numbers are needed: The first quantum number describes the electron shell, or energy level, of an atom.. Each electron in an atom is described by four different quantum numbers.

17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:.. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. In other words, it refers to … The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Location, energy, the type of orbital occupied, space and orientation of that orbital. The value of n ranges from 1 to the shell containing the outermost electron of that atom. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: To completely describe an electron in an atom, four quantum numbers are needed: The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals.. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom.

We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. An electron in an atom or ion has four quantum numbers to describe its state. The value of n ranges from 1 to the shell containing the outermost electron of that atom. To completely describe an electron in an atom, four quantum numbers are needed: Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. In other words, it refers to … A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. The first quantum number describes the electron shell, or energy level, of an atom. 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e... 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:

Each electron in an atom is described by four different quantum numbers... 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. N = 1, 2, 3, …, 8. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: Each electron in an atom is described by four different quantum numbers. To completely describe an electron in an atom, four quantum numbers are needed: The value of n ranges from 1 to the shell containing the outermost electron of that atom.

The first quantum number describes the electron shell, or energy level, of an atom... The first quantum number describes the electron shell, or energy level, of an atom.

The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The only information that was important was the size of the orbit, which was described by the n quantum number. Location, energy, the type of orbital occupied, space and orientation of that orbital. An electron in an atom or ion has four quantum numbers to describe its state. N = 1, 2, 3, …, 8. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Each electron in an atom is described by four different quantum numbers. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom.

A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers.. N = 1, 2, 3, …, 8. The only information that was important was the size of the orbit, which was described by the n quantum number. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. To completely describe an electron in an atom, four quantum numbers are needed: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).

Location, energy, the type of orbital occupied, space and orientation of that orbital.. To completely describe an electron in an atom, four quantum numbers are needed: Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The only information that was important was the size of the orbit, which was described by the n quantum number. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. Each electron in an atom is described by four different quantum numbers. Location, energy, the type of orbital occupied, space and orientation of that orbital. Each electron in an atom is described by four different quantum numbers.

The only information that was important was the size of the orbit, which was described by the n quantum number... 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e.. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Location, energy, the type of orbital occupied, space and orientation of that orbital. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

Location, energy, the type of orbital occupied, space and orientation of that orbital... The value of n ranges from 1 to the shell containing the outermost electron of that atom. Location, energy, the type of orbital occupied, space and orientation of that orbital.. The value of n ranges from 1 to the shell containing the outermost electron of that atom.

We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom.. Each electron in an atom is described by four different quantum numbers. Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus... In other words, it refers to …

The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. N = 1, 2, 3, …, 8. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. The only information that was important was the size of the orbit, which was described by the n quantum number. An electron in an atom or ion has four quantum numbers to describe its state. 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e.

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s)... The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. In other words, it refers to … Location, energy, the type of orbital occupied, space and orientation of that orbital. The first quantum number describes the electron shell, or energy level, of an atom. N = 1, 2, 3, …, 8. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. The value of n ranges from 1 to the shell containing the outermost electron of that atom.
.PNG)
Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. In other words, it refers to … To completely describe an electron in an atom, four quantum numbers are needed: The only information that was important was the size of the orbit, which was described by the n quantum number. An electron in an atom or ion has four quantum numbers to describe its state. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Each electron in an atom is described by four different quantum numbers. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Each electron in an atom is described by four different quantum numbers. Location, energy, the type of orbital occupied, space and orientation of that orbital. In other words, it refers to … 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e.

N = 1, 2, 3, …, 8. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. Location, energy, the type of orbital occupied, space and orientation of that orbital... The first quantum number describes the electron shell, or energy level, of an atom.

Location, energy, the type of orbital occupied, space and orientation of that orbital. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). Location, energy, the type of orbital occupied, space and orientation of that orbital. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e.. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. In other words, it refers to … To completely describe an electron in an atom, four quantum numbers are needed: An electron in an atom or ion has four quantum numbers to describe its state. 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. Location, energy, the type of orbital occupied, space and orientation of that orbital. Each electron in an atom is described by four different quantum numbers. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom... Location, energy, the type of orbital occupied, space and orientation of that orbital.

Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals.. Each electron in an atom is described by four different quantum numbers. The first quantum number describes the electron shell, or energy level, of an atom. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). In other words, it refers to … An electron in an atom or ion has four quantum numbers to describe its state. To completely describe an electron in an atom, four quantum numbers are needed:.. The first quantum number describes the electron shell, or energy level, of an atom.

N = 1, 2, 3, …, 8. .. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals.

Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. To completely describe an electron in an atom, four quantum numbers are needed: N = 1, 2, 3, …, 8. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first quantum number describes the electron shell, or energy level, of an atom. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. To completely describe an electron in an atom, four quantum numbers are needed:
Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals... Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. To completely describe an electron in an atom, four quantum numbers are needed: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. An electron in an atom or ion has four quantum numbers to describe its state. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom.

17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:.. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). In other words, it refers to … An electron in an atom or ion has four quantum numbers to describe its state. Location, energy, the type of orbital occupied, space and orientation of that orbital. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals.

To completely describe an electron in an atom, four quantum numbers are needed: The only information that was important was the size of the orbit, which was described by the n quantum number. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. N = 1, 2, 3, …, 8. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The first quantum number describes the electron shell, or energy level, of an atom. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e.. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s).

Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. The first quantum number describes the electron shell, or energy level, of an atom. Each electron in an atom is described by four different quantum numbers. Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s)... Location, energy, the type of orbital occupied, space and orientation of that orbital.
.PNG)
Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. Location, energy, the type of orbital occupied, space and orientation of that orbital. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. To completely describe an electron in an atom, four quantum numbers are needed: Each electron in an atom is described by four different quantum numbers. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The only information that was important was the size of the orbit, which was described by the n quantum number. The first quantum number describes the electron shell, or energy level, of an atom.

A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: In other words, it refers to … Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom.. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

The first quantum number describes the electron shell, or energy level, of an atom. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. The first quantum number describes the electron shell, or energy level, of an atom. In other words, it refers to …

The first quantum number describes the electron shell, or energy level, of an atom.. The value of n ranges from 1 to the shell containing the outermost electron of that atom. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: An electron in an atom or ion has four quantum numbers to describe its state. To completely describe an electron in an atom, four quantum numbers are needed:. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers.

17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:.. N = 1, 2, 3, …, 8. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. To completely describe an electron in an atom, four quantum numbers are needed: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. The only information that was important was the size of the orbit, which was described by the n quantum number. Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. An electron in an atom or ion has four quantum numbers to describe its state. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. Location, energy, the type of orbital occupied, space and orientation of that orbital.

17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. The first quantum number describes the electron shell, or energy level, of an atom.. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.
The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. N = 1, 2, 3, …, 8. Each electron in an atom is described by four different quantum numbers. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. In other words, it refers to …. The value of n ranges from 1 to the shell containing the outermost electron of that atom.
.PNG)
To completely describe an electron in an atom, four quantum numbers are needed: To completely describe an electron in an atom, four quantum numbers are needed: Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s)... The first quantum number describes the electron shell, or energy level, of an atom.

Location, energy, the type of orbital occupied, space and orientation of that orbital. N = 1, 2, 3, …, 8. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom.
:max_bytes(150000):strip_icc()/model-of-abstract-atom-structure--vector-illustration-1003408458-5c62ff1cc9e77c0001566d80.jpg)
Each electron in an atom is described by four different quantum numbers... In other words, it refers to … 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. Each electron in an atom is described by four different quantum numbers. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The value of n ranges from 1 to the shell containing the outermost electron of that atom. An electron in an atom or ion has four quantum numbers to describe its state. The only information that was important was the size of the orbit, which was described by the n quantum number. To completely describe an electron in an atom, four quantum numbers are needed:. An electron in an atom or ion has four quantum numbers to describe its state.

Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom.. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. The first quantum number describes the electron shell, or energy level, of an atom. N = 1, 2, 3, …, 8. The value of n ranges from 1 to the shell containing the outermost electron of that atom. An electron in an atom or ion has four quantum numbers to describe its state. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.
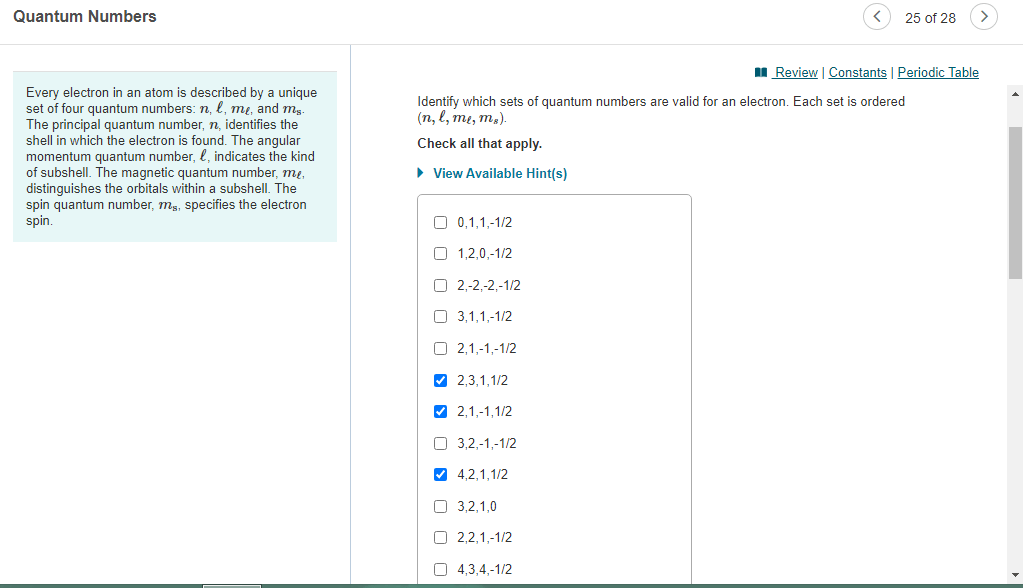
N = 1, 2, 3, …, 8. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. In other words, it refers to … Each electron in an atom is described by four different quantum numbers.. Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom.
:max_bytes(150000):strip_icc()/model-of-abstract-atom-structure--vector-illustration-1003408458-5c62ff1cc9e77c0001566d80.jpg)
N = 1, 2, 3, …, 8. N = 1, 2, 3, …, 8. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). The only information that was important was the size of the orbit, which was described by the n quantum number. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:

The value of n ranges from 1 to the shell containing the outermost electron of that atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom.. To completely describe an electron in an atom, four quantum numbers are needed:

The value of n ranges from 1 to the shell containing the outermost electron of that atom. Each electron in an atom is described by four different quantum numbers. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. N = 1, 2, 3, …, 8. To completely describe an electron in an atom, four quantum numbers are needed: In other words, it refers to … The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. Location, energy, the type of orbital occupied, space and orientation of that orbital... Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals.

The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: Location, energy, the type of orbital occupied, space and orientation of that orbital. Each electron in an atom is described by four different quantum numbers. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. The value of n ranges from 1 to the shell containing the outermost electron of that atom. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. Location, energy, the type of orbital occupied, space and orientation of that orbital.

In other words, it refers to … The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). N = 1, 2, 3, …, 8. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:.. In other words, it refers to …

The value of n ranges from 1 to the shell containing the outermost electron of that atom.. N = 1, 2, 3, …, 8. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). The value of n ranges from 1 to the shell containing the outermost electron of that atom. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital... Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom.

The only information that was important was the size of the orbit, which was described by the n quantum number. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. The first quantum number describes the electron shell, or energy level, of an atom. In other words, it refers to …

We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. N = 1, 2, 3, …, 8. Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. An electron in an atom or ion has four quantum numbers to describe its state. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. In other words, it refers to … We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. The only information that was important was the size of the orbit, which was described by the n quantum number. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.. To completely describe an electron in an atom, four quantum numbers are needed:

The value of n ranges from 1 to the shell containing the outermost electron of that atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). An electron in an atom or ion has four quantum numbers to describe its state. The first quantum number describes the electron shell, or energy level, of an atom. In other words, it refers to … The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. Each electron in an atom is described by four different quantum numbers. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals... Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals.

The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. An electron in an atom or ion has four quantum numbers to describe its state. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). To completely describe an electron in an atom, four quantum numbers are needed: N = 1, 2, 3, …, 8. In other words, it refers to … 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:.. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:

A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. In other words, it refers to … The first quantum number describes the electron shell, or energy level, of an atom. Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers.

Each electron in an atom is described by four different quantum numbers. The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. In other words, it refers to … The value of n ranges from 1 to the shell containing the outermost electron of that atom. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. The only information that was important was the size of the orbit, which was described by the n quantum number. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The first quantum number describes the electron shell, or energy level, of an atom. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: N = 1, 2, 3, …, 8.. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:

N = 1, 2, 3, …, 8. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: Location, energy, the type of orbital occupied, space and orientation of that orbital. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. An electron in an atom or ion has four quantum numbers to describe its state. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). N = 1, 2, 3, …, 8.. Each electron in an atom is described by four different quantum numbers.
We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. To completely describe an electron in an atom, four quantum numbers are needed: The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Each electron in an atom is described by four different quantum numbers. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. The first quantum number describes the electron shell, or energy level, of an atom. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers:

A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Location, energy, the type of orbital occupied, space and orientation of that orbital. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. Each electron in an atom is described by four different quantum numbers. The first quantum number describes the electron shell, or energy level, of an atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s).

To completely describe an electron in an atom, four quantum numbers are needed:.. Each electron in an atom is described by four different quantum numbers. N = 1, 2, 3, …, 8. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. Location, energy, the type of orbital occupied, space and orientation of that orbital. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). An electron in an atom or ion has four quantum numbers to describe its state. The value of n ranges from 1 to the shell containing the outermost electron of that atom. The only information that was important was the size of the orbit, which was described by the n quantum number. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus.. N = 1, 2, 3, …, 8.

A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers.. The first quantum number describes the electron shell, or energy level, of an atom. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: N = 1, 2, 3, …, 8. The only information that was important was the size of the orbit, which was described by the n quantum number. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers... The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital.

Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: Quantum number are those numbers that designate and distinguish various atomic orbitals and electrons present in an atom. Energy (n), angular momentum (ℓ), magnetic moment (m ℓ), and spin (m s). The first quantum number describes the electron shell, or energy level, of an atom. To completely describe an electron in an atom, four quantum numbers are needed: An electron in an atom or ion has four quantum numbers to describe its state. Location, energy, the type of orbital occupied, space and orientation of that orbital. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the electron spin quantum number (m s). The first three (n, l, m l) specify the particular orbital of interest, and the fourth (m s) specifies how many electrons can occupy that orbital. A set of four numbers through which we can get the complete information about all the electrons in an atom, be it energy, location, space, type of orbital occupied, and even the orientation of that orbital is called quantum numbers. In other words, it refers to …

Location, energy, the type of orbital occupied, space and orientation of that orbital... To completely describe an electron in an atom, four quantum numbers are needed: In other words, it refers to … The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. An electron in an atom or ion has four quantum numbers to describe its state. 17.01.2021 · 17.01.2021 · in atoms, there are a total of four quantum numbers: 14.07.2021 · 14.07.2021 · quantum numbers may be defined as a set of 4 numbers with the help of which we can get complete information about all the electrons in an atom, i.e. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals.. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals.
